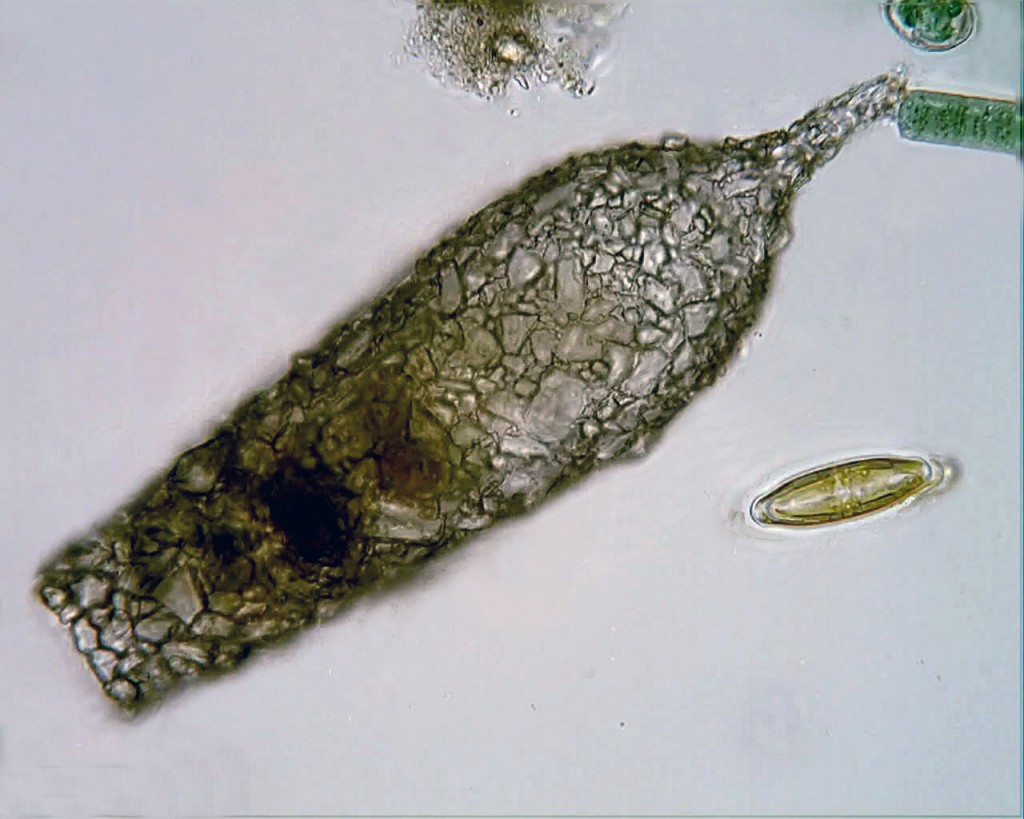
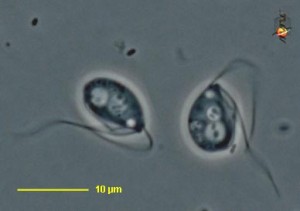
On a recent trip to the eastern coast of James Bay, I collected large numbers of testate amoebae that, by morphological criteria, belong to various species in the genus Difflugia. I’ve spent some time making portraits of them, measuring them, and dropping live ones in little vials of guanidinium thiocyanate in the hope of eventually getting a gene sequence from them. My plan is to figure out how some of these Difflugia-shaped things relate to one another, and to other Difflugia-shaped things that have already been sequenced, and then to compare all of these to certain Difflugia-shaped things that have been described in the past.
But before getting to that, I needed to get up to speed on the tangled taxonomic history of the Difflugia, the oldest genus in Arcellinida. It’s a task that calls for a certain kind of person, one who enjoys rummaging through centuries-old texts, and squinting at bad scans of fading illustrations, to settle really small arguments about really small creatures.
In other words: a pedant.
I’ll need to draw on deep reserves of pedantry, here. I won’t apologize for that, but I can include a warning, at least: to anyone not already obsessed with the subject–as I am, obviously–it will be dull. Numbingly, crushingly, excruciatingly dull.
The beginning
Sometime in the early 1800s, a French microscopist named Léon Leclerc encountered a creature like none that had ever been described. It was no bigger than a tenth of a French “ligne”, which would make it about 226 micrometres long. It lived in a little shell covered with grains of sand. From the mouth of this “têt” (test) it extended “long arms of a beautiful milky white” which varied constantly in size, number and organization.
At first he took it for a small mollusc, and tried unsuccessfully to find its eyes. He also tried to see cilia, like those found in other “animalcules,” but it had none. In subsequent investigations, he encountered thousands more of them in a variety of types, but he could never make out any internal organs or determine what food they ate.
Because of the “imperfection” of his observations, he waited a long time to announce his discovery to the world. Finally, in 18161, at the urging of the horticulturalist Louis Bosc (best known, maybe, for the sweet pear that bears his name), he published a short article describing a new genus of “amorphous polyp.” He called it “la Difflugie,” or “Difflugia.”
This was the origin of a classificatory hairball that taxonomists are still trying to cough up, more than two hundred years later.
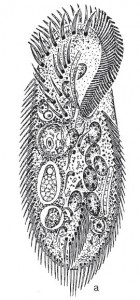
In his article, Leclerc included six figures depicting what appear to be three species of arcellinid testate amoebae.2 The first (labelled F. 1 and F.1a) is easily identifiable as Lesquereusia modesta, the morphospecies I discussed in my last blog post. The second (labelled F. 2 and F. 3) is roughly pear-shaped, and possibly a member of what we now call the Difflugia pyriformis complex (like the one in my SEM image, above). The third one (F. 5) is usually interpreted as Difflugia acuminata, a species that has recently been moved to another genus in a clade that branches well apart from Difflugia.
Leclerc did not name any of the species he depicted, and he did not assign a type species to his genus, as modern taxonomists are expected to do.
This lapse was remedied the following year, when the great French zoologist Lamarck added Leclerc’s Difflugia to his colossal multi-volume Histoire naturelle des animaux sans vertèbres. Lamarck proposed that the type species of the genus should be called Difflugia protæiformis. Unfortunately, he did not supply a picture or description of this new species, but simply referred to Leclerc’s “mss” (presumably, the manuscript version of the paper Leclerc published). So, which of Leclerc’s drawings is Lamarck’s D. protæiformis? The answer seems to be: all of them. His decision to combine three different forms under one name is perhaps explained by the specific epithet he chose, “protæiformis“, that is, “protean in form” (mutable, ever-changing). It is possible that the term refers not only to the perpetually shifting organism itself but also the presumed variability of its shell.
This means that, for the purposes of taxonomy, the “type species” of Difflugia is a chimeric entity consisting of three different species, two of which are no longer even included in the genus.
C.G. Ehrenberg
In 1830, the great German zoologist C. G. Ehrenberg incorporated Difflugia into his classification of “infusion animals”, placing it in the group of shelled “Schmelzthierchen” (“melt animals”) he called Arcellina. At first, he recognized just two members of the genus: the type species, which he spelled “D. proteiformis” (dropping the ligature Lamarck had included in the name), and another one that had a long shell equipped with a spike, which he called “the pointed melt animal,” Difflugia acuminata.3 Both species were given full descriptions in his magnum opus of 1838.
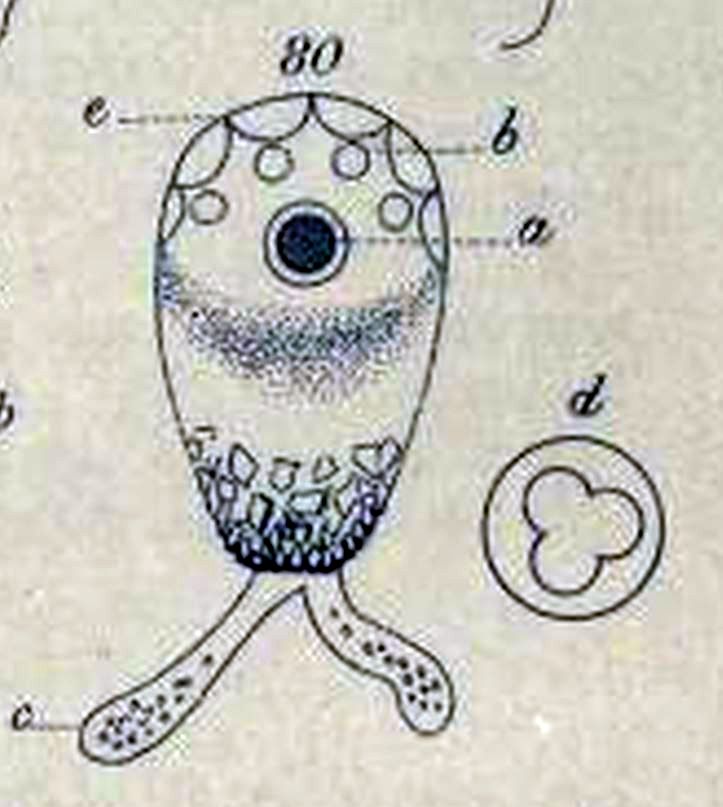
The various forms he identifies as proteiformis in that later work are all quite different from any of the Difflugia in Leclerc’s illustrations. They are most similar to Leclerc’s F. 2,3 but to modern eyes none of them look like the same species. On the figure to the left, the one he labelled “I. c” has a lobed aperture, so it is likely a species of Netzelia, such as N. lobostoma or N. gramen. The ones labelled “I.a” and “I.b” have an overall shape and texture that suggests Netzelia tuberculata, to me. The other two (“l.d” and “l.e”) are unrecognizable, but both much smaller, and rather different from one another.
So, Ehrenberg’s proteiformis, like Leclerc’s, appears to be a chimeric entity, combining three or four morphotypes under one name.
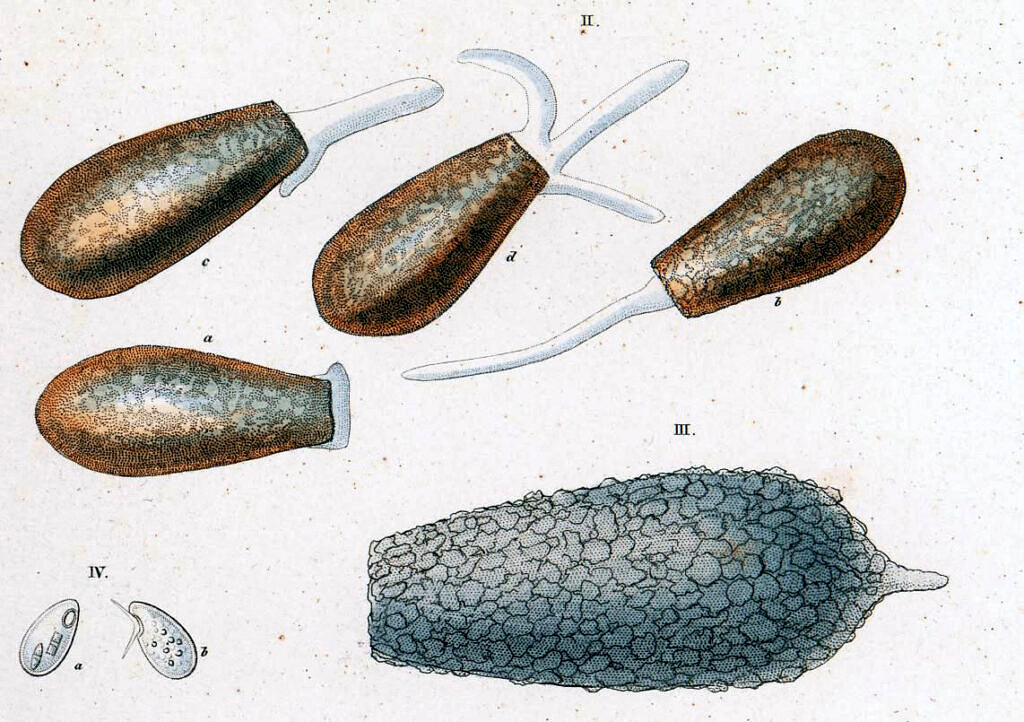
The second species he found and named in 1830, Difflugia acuminata (the large, pointed shell labelled III in the drawing to the right), was probably related to the one Leclerc depicted in his third drawing (Pl. 17, Fig. 5). In 1838, Ehrenberg added another large species to the genus, which he called Difflugia oblonga (II a-d in the illustration to the right).
Both of these two species would play a role in the decades of taxonomic confusion that would ensue.
Maximilian Perty
The next to revise the genus was Maximilian Perty, professor of zoology at Bern University in Switzerland. In 1849, Perty published a survey of the microscopic organisms of the Italian Swiss Alps. Among the species of Difflugia he found in alpine lakes, he mentions “D. Proteus“, probably an idiosyncratic rendering of D. proteiformis. Three years later, he published an illustration of that species. The specimen he depicts is one he considered a “Monstrosität”, a deformity of Difflugia proteiformis. It is easily recognized as the notably non-monstrous species we call Lesquereusia spiralis.
In addition, he names two new species: Difflugia acaulis (a lanceolate shell, which he would later describe as a variety of Ehrenberg’s D. acuminata); and one to which he gives the name Difflugia pyriformis. The latter is described by Perty as “pear-shaped”, with its narrower end toward the mouth and a texture like that of D. proteiformis Ehrenberg. Perty gives its size as 1/7-1/5”’.4
The unit he is using there is the “line”, the exact size of which varies from one European locality to the next. It is usually one twelfth of whatever length the local inch happened to be. We don’t know exactly what version of the unit Perty was using, but a reasonable guess would be the Swiss line, which is the same as the French one, equal to 2.26 mm. So, Perty’s pyriformis probably ranged from 323 μm to 452 μm in length.
It’s a big shell!
This is worth emphasizing, because later authors would conflate the species with Ehrenberg’s D. oblonga. That species, however, was only 1/18th of a line long, which comes to somewhere between 111 and 125 μm (depending on whether he was using the Prussian, the Viennese or the French line).5 So, Perty’s new species, though somewhat similar in shape to Ehrenberg’s, is about three times bigger. Yet, despite the size disparity, even Perty himself seems to have some difficulty differentiating the two species, expressing doubts about eight specimens he had gathered in Switzerland’s Rosenlaui gorge and identified as D. oblonga: “Could they have belonged to my D. pyriformis?” (my translation).6
Joseph Leidy
The next major figure on our Mount Rushmore of Difflugia taxonomy is Joseph Leidy, author of the most beautiful book ever written about amoebae: Fresh-water Rhizopods of North America (1879). Leidy puts his finger on the problem with the type species. “The name of Difflugia proteiformis,” he reminds us, “is exceedingly indefinite in its application.”
For some of his contemporaries, the “indefinite application” of the name was actually the point. It reflected what they took to be the extreme variability of that species. One of them, the cranky and quarrelsome George Charles Wallich, took this notion to its limit, arguing that that all the named varieties of Difflugia–and those of Arcella, too, as well as Centropyxis, Nebela, Quadrulella and some others–were just different forms of Difflugia proteiformis, and that variations in their shells were only the result of “the ever-changing fluctuations of the medium by which the organisms are surrounded”. 7
Leidy–who was himself a taxonomic “lumper”, by modern standards–concedes that the Difflugia shell was “very variable in shape”, but sorts these variations into 10 distinct species. He does what he can to disentangle proteiformis from some of the forms to which it had already been attached.
Leidy recognizes that Lamarck’s species name did not apply to any one of Leclerc’s drawings, but to all of them together “without discrimination”; and further, that all three could be identified with species that had been described by later authors under other names: D. spiralis, D. acuminata and D. pyriformis sensu Perty.
Additionally, he recognized that Ehrenberg’s proteiformis was something different from any of those in Leclerc’s drawings. He considers it a synonym of another Difflugia, a balloon-shaped arcellinid with a round aperture first described by Felix Dujardin, in 1837, as D. globulosa. Dujardin himself clearly distinguished his species from D. proteiformis, since the latter had a shell “covered with little grains of sand,” whereas his globulosa was smooth.8 However, Leidy’s version of globulosa–based on specimens he had gathered in North America–had a sand-covered shell, so it could be comfortably synonymized with Ehrenberg’s proteiformis, regardless of whether it was the same as Dujardin’s.9
But, lest anyone think the case had been solved, he introduced another twist to the plot. In a later section of his book, Leidy invokes a different candidate for Ehrenberg’s proteiformis, a species with a three-lobed aperture which he himself had described under the name Difflugia lobostoma. Of that species, he writes: “As ordinarily seen, it bears so close a resemblance with the corresponding views of Difflugia proteiformis, as described and figured by Ehrenberg, that it may not only be readily taken for the same, but I have suspected that Ehrenberg may have actually had this animal under observation when he described D. proteiformis.”
Leidy suspects his own lobostoma to be identical with a species that had been described two decades earlier by H. J. Carter, as Difflugia tricuspis, but he rejects that name–despite its priority–because “tricuspis” implies that this species could only have three lobes in its aperture, whereas Leidy had determined that it could have as many as six.10
Whatever name we assign to it, Leidy’s Difflugia lobostoma, strikes me as a reasonable candidate for at least one of Ehrenberg’s proteiformis illustrations. As I mentioned above, Ehrenberg’s Fig. Ic clearly shows a lobed aperture.11 However, the size Ehrenberg gives (100 μm) falls in a grey zone between Netzelia lobostoma and Netzelia gramen. In any case, as I pointed out before, there’s no particular reason to feel sure that Ehrenberg’s illustrations all depict the same species.
Eugène Penard & the pyriformis problem
In 1902, the genus Difflugia went pear-shaped, when Eugène Penard reassigned Lamarck’s type species to D. pyriformis Perty, 1852. That placement–based only on Leclerc’s Fig. 2 & Fig. 3–is arguably more plausible than Leidy’s (D. globulosa), but it steers the taxonomy directly into yet another collision.
In 1909, in the second volume of their British Freshwater Rhizopoda, James Cash and his assistant John Hopkinson conducted an exhaustive literature review of Difflugia pyriformis Perty (1852) and Difflugia oblonga Ehrenberg (1838). The bibliographic effort was magisterial–their references fill more than three closely-packed pages–and their final judgment was expressed very simply: the two species were one, and since Ehrenberg’s had priority they should be collapsed together under the name he used. Henceforth, everything known D. pyriformis would be called oblonga.
As for the type species, our old friend “Difflugia proteiformis,” that presented a problem. If it were retained as D. pyriformis, that would make it a synonym of D. oblonga, in which case Ehrenberg’s two species–and all the observations that had ever been referred to them–would be compressed together into one misshapen lump. However, Ehrenberg’s oblonga and proteiformis, according to Cash and Hopkinsons, could not be accepted as synonyms. Therefore, they wrote, “the safest course now is to discard the name proteiformis altogether.”
The proposed synonymy of pyriformis and oblonga made it a remarkably variable (one might even say “protean”!) species. As Ferry Siemensma has pointed out, in a very useful discussion on the D. oblonga page of his website, there is a rather spectacular size difference between the species Ehrenberg illustrated and the pyriformis morphotype as it is generally understood. It’s the kind of difference that is easily overlooked when we are looking only at illustrations and photos in published sources, so Ferry dramatizes it for us by providing a properly scaled picture of Ehrenberg’s shell alongside a group of shells illustrated in Chardez (1967) as varieties of D. oblonga:

If you were to encounter a group like this in your microscope would you consider the little straight-sided shell on the left to be a member of the same morphospecies as the large bottle-shaped ones on the right?
I would not.
However, that’s a subjective judgment. To prove that they truly are different would require a better method than my usual one of peering at them thoughtfully while stroking my chin.
Palaeontology weighs in
When Cash and Hopkinson recommended discarding proteiformis, they just formalized what taxonomists had already been doing: ignoring an incoherent taxon. For the purposes of stable classification, though, this was not ideal.
In taxonomy, the type species of a genus has a special status: it’s the indispensable thing to which the genus name refers. Other species can be removed from the genus, but the type species is permanently tied to it. Without its type species, the validity of the genus itself comes into question.

In 1964, this situation was addressed by two American micropalaeontologists (who happened to be married to one another), Alfred. R. Loeblich and Helen Tappan. By that time, Difflugia and its 300 nominal species had been drifting along without a clear type species for nearly five decades. As others had before, Loeblich and Tappan noted that Lamarck had never specified which of Leclerc’s drawings should represent the type of the species. So, as Leidy and Penard had also done, they picked one of Leclerc’s images to fill this position. The illustration Loeblich and Tappan chose was Leclerc’s Fig. 5, the one with a pointed fundus, which is usually regarded as a specimen of Ehrenberg’s D. acuminata.
Their reason for picking Fig. 5 was interesting, and (I think) novel. In their interpretation, all the other images Leclerc had drawn (Figs. 1-4) were depictions of the same organism, the one now known as Lesquereusia. As they saw it, the drawings that look like “pyriformis” (Figs. 2 and 3) were simply “edge views” of the first two. So, by their reckoning, Leclerc’s Fig. 5 was the only one of his drawings that was still “unquestionably Difflugia as generally understood.”
They formally declared this image to represent the type species of the genus: “As no lectotype has yet been designated, we here designate as lectotype of D. protoeiformis the specimen illustrated on Pl. 17 Fig. 5 of Leclerc.”
Their spelling of the species name was a simple mistake–they seem to have misread the aesc ligature (æ) used by Lamarck as an oethel (œ). Later authors would restore Lamarck’s original spelling (but with no ligature, since that is forbidden under ICZN rules).
Their term “lectotype” refers to the use of an illustration to serve as the “name-bearing type” of the species. In taxonomy, every species is expected to be based on a single representative specimen. Ideally, this would be a material object, a preserved specimen which acts as a permanent reference point for the taxon. For obvious reasons, physically preserved “holotypes” of microscopic species (especially older ones) are often unavailable. In such cases, the rules of nomenclature allow an illustration or photograph to serve the same purpose.
Their choice of Leclerc’s fifth figure as the name-bearing type of Difflugia meant that Ehrenberg’s Difflugia acuminata would now be a junior synonym of Difflugia protaeiformis. A lot of work had already been done on that morphotype under Ehrenberg’s name, so it was a bit awkward. And, of course, the resurrection of D. protaeiformis risked reviving the taxonomic chaos that had swirled around that name from the beginning.
By and large, researchers who have accepted Loeblich and Tappan’s lectotype have come from the community of palaeontologists and palaeolimnologists, many of whom use fossil testate amoebae as indicators of ecological conditions in ancient bodies of water. Those who come to the subject from protistology, eukaryotic microbiology, cell biology, etc., have generally rejected or ignored it.
In 1988, Ogden & Ellison questioned the validity of Loeblich and Tappan’s lectotype, on the basis of their personal communication with a colleague identified as “Merifield” who argued that, as he interpreted the International Code of Zoological Nomenclature (ICZN), Article 74b, the image that had been chosen for their lectotype was “invalid and, as a consequence, should be rejected.” They went on to promise that the matter would soon be brought before the ICZN: “A complete justification of this opinion is in preparation (Merifield, in prep.) for submission to the Commission, in which it is hoped to designate a more correct type species for Difflugia.”
I don’t know what became of this plan, but if a “more correct type species” was ever designated, I can find no reference to it.
Deconstructing Difflugia, 2022
Since that episode, Difflugia systematics has moved on. Between 2012 and 2015, in a series of three papers, Yuri Mazei and Alan Warren undertook an ambitious review of the genus, based on shells in collections left by Eugene Penard and Colin G. Ogden. They left Loeblich and Tappan out of the discussion, and allowed the question of the type species to remain unsettled, but that made little difference because by this time arcellinid taxonomy was already well into the era of molecular phylogenetics.
In 2022, an all-star team of testate amoeba specialists headed by Ruben González-Miguéns investigated some putative members of Difflugia by looking at certain mitochondrial and nuclear genes. Their findings confirmed earlier indications that the old genus was a polyphyletic grouping, which is to say that some of its members are more closely related to arcellinids in other groups than they are to each other.
As a first step toward recovering all the monophyletic groups currently buried in Difflugia, they carved some new taxa from the old genus. One is a single-species genus they named Golemanskia, in honor of a colleague. The other comprises a handful of “Difflugia” species, mostly with long and/or pointy shells. For that one they came up with a jaunty portmanteau, combining “cylindrical” and “Difflugia“: Cylindrifflugia.

Among the species they transferred to Cylindrifflugia was none other than Leclerc’s “fig. 5”, Difflugia acuminata. Consequently, the oldest “surviving” member of Difflugia (and, according to some, its type species) is now in a separate genus. The genus itself now resides in the new infraorder Cylindrothecina, a group delimited “by its specific sequences of the mitochondrial and nuclear DNA markers (COI, NADH and SSU) and by its phylogenetic placement.”
Specimens identified as D. pyriformis, nodosa and oblonga group well apart from Cylindrifflugia, and are retained in the infraorder Longithecina where, presumably, they will make up the core difflugiids in future investigations, as more taxa are carved from the old genus.
At some point, some brave taxonomist will have to propose a type species for the ones that remain.
Notes
- The date is usually given as 1815, but that was corrected by Loeblich and Tappan, in 1964. The volume in which Leclerc’s article appears carries the date 1815, but includes publications issued over two years. ↩︎
- However, Loeblich and Tappan read the first four images as belonging to a single species, as I’ll explain further on. ↩︎
- Difflugia acuminata was discovered as Ehrenberg’s book of 1830 was in already in press, and he gives little information about it, though he does specify that the creature “disdains coloured food.” ↩︎
- The size range is taken from his book of 1852. In the original description, in 1849, he gives a slightly narrower range of 1/6-1/5”’. ↩︎
- This has been pointed out by Ferry Siemensma on his website. ↩︎
- Mazei and Warren misread this passage, taking it to mean that Perty is questioning the validity of Ehrenberg’s oblonga. It’s a puzzling conjecture, since oblonga clearly had priority and Perty would not have thought his new name would supercede the older one. He doesn’t appear to have any doubts about the validity of either oblonga or his pyriformis, but just doesn’t know where to assign his Rosenlaui specimens. ↩︎
- For clarity, I’m mostly omitting Wallich’s contributions, but they’re quite interesting. He published a detailed revision of the Difflugidae in 1864, seemingly based on his belief that shell shapes grow and change as amoebae mature. From this starting place, he argues that all the named varieties of Difflugia–and those of Arcella, too, as well as all the amoebae we now place in Centropyxis, Nebela, Quadrulella etc.–were a single species, and that variations in the shells were only the result of “the ever-changing fluctuations of the medium by which the organisms are surrounded”. There is much to be said about this unusual gentleman, but I’ll reserve it for another day. His ideas about amoeboid classification were not very influential. ↩︎
- Dujardin’s description of D. proteiformis: “An[imale] à coque noirâtre ou verdâtre, globuleuse ou ovoïde, recouverte de petits grains de sable. — Longueur 0,043 à 0,112.” And his description of D. globulosa: “An[imale] à coque brune, globuleuse ou ovoïde , lisse. — Longueur 0,10 à 0,25.” ↩︎
- I should point out another uncomfortable fact, which is that neither Dujardin’s D. globulosa nor the one depicted by Leidy actually resemble the organism conventionally given that name in modern work. In recent work, the name D. globulosa is applied to species that are nearly spherical, with a much wider aperture than we see in Dujardin’s image of 1837. The modern idea of globulosa resembles the arcellinid Wallich recorded as Difflugia globularis, which some taxonomist still differentiate from globulosa. Leidy takes Wallich’s name to be an erroneous spelling of D. globulosa, and Wallich himself does not dispute that claim in his furiously detailed critique of Leidy’s book. See: “Critical Observation on Joseph Leidy’s Fresh-water Rhizopods of North America” (1885). Ogden examined Wallich’s annotated copy of Leidy, and reported that Wallich had written “quite true, it was a mistake” in the margins, next to Leidy’s comments (Ogden, 1988). ↩︎
- Under the rules that govern nomenclature, Leidy’s D. lobostoma is really a junior synonym of D. tricuspis Carter, 1856 (currently, Netzelia lobostoma). However, Leidy’s name is the one everybody uses, and…well, the taxonomy is already complicated enough! ↩︎
- Cash, Wailes and Hopkinson agree with this interpretation, saying that Ehrenberg’s proteiformis was “in all probability one of the species with a lobate mouth (e.g. D. lobostoma Leidy).” ↩︎